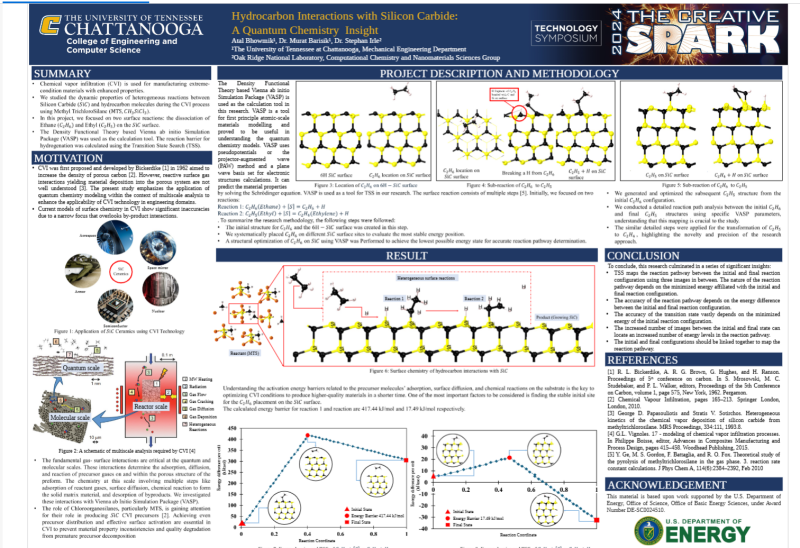
Chemical vapor infiltration (CVI) is used for manufacturing extreme-condition materials with enhanced properties. However, reactive surface gas interactions yielding material deposition into the porous system are not well-understood. As a part of our ongoing Department of Energy (DOE) project, we studied the dynamic properties of heterogeneous reactions between silicon carbide (SiC) and various hydrocarbon molecules during the CVI process. The precursors generated by the thermal breakdown of methyl trichlorosilane (MTS, CH3SiCl3) were studied in this work. The surface reaction consists of multiple steps. In the beginning, we focused on two reactions: the dissociation of Ethane and ethylene on the SiC surface. The Density Functional Theory-based Vienna ab initio Simulation Package (VASP) was used as the calculation tool. The reaction barrier for hydrogenation was calculated using the Transition State Search. Understanding the activation energy barriers related to the precursor molecules' adsorption, surface diffusion, and chemical reactions on the substrate is the key to optimizing CVI conditions to produce higher-quality materials in a shorter time.
Add comment
Comments